How to Size Gas or Steam Motive Eductors for Evacuating Gases
 Using the Eductor Models SG and HG
Using the Eductor Models SG and HG
Priming
Priming is simply a special type of evacuation where the reduction of pressure in the vessel is used to draw liquid into the vessel. A good rule of thumb for priming applications is that they take twice as long as an evacuation for the same volume and pressure.
Step 1 Before beginning to do the actual sizing, convert all pressures and flows to the units used in the SG, HG sizing table below.
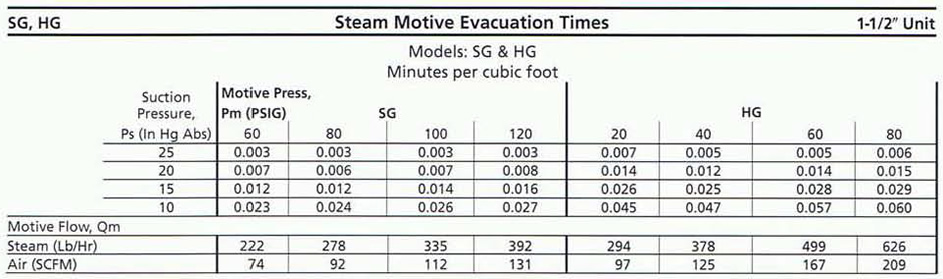
Step 2 After determining the required suction pressure to be achieved, divide the desired time to achieve this vacuum by the number of Ft3 to be evacuated. This will result in a time per Ft3 to perform the evacuation.
Desired Evacuation Time/Ft3 to be Evacuated = Desired Time Per Ft3
Step 3 Find the column from the performance table that corresponds to your motive pressure. Find the row with the desired suction pressure on the left-hand side of the performance table. By dropping down to this intersection, the time for evacuating I Ft3 in minutes is found for the 1-1/2" unit.
Step 4 Take the tabulated time per Ft3 found in Step 3 and divide it by the desired minutes per Ft) found in Step 2. The result will be a Desired Sizing Factor (S.F.). Go to the Table (below) and pick the unit that will meet or exceed the factor found above.
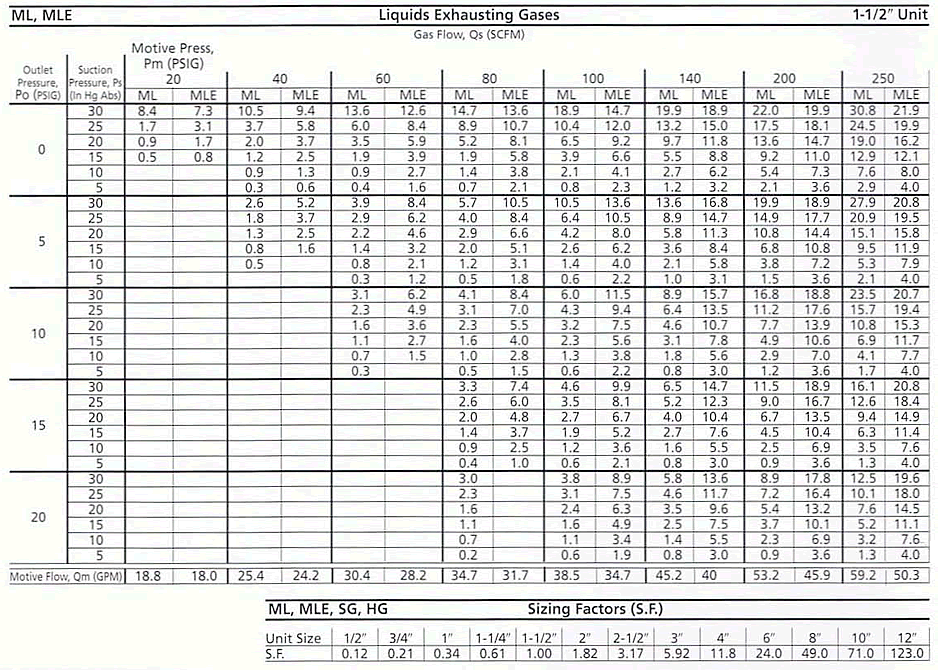
Step 5 Divide the tabulated time per Ft) by the S.F. of the unit selected. Multiply this by the number of Ft3 to be evacuated. The result will be the actual time required to evacuate the vessel with the eductor selected.
Step 6 To determine the motive flow required, go to the chart for the motive gas you are using. Then find the motive pressure you are using under this column and locate the model of unit you are using. Multiply this flow by the S.F. of the unit you selected in Step 4.
Note: The models SG and HG are sized using the same steps as the ML and MLE; the only difference is the motive force is provided by steam or gas.
- Example:
Area to be Evacuated = 35 Ft3
Time to Evacuate = 12 minutes
Desired Suction Pressure (Ps) = 15 In Hg Abs
Gas to be Removed = Air
Motive Gas = Steam
Motive Pressure (Pm) = 60 PSIG
Outlet Pressure (Po) = Atmospheric Pressure
- Step 1 No conversions are needed as all units are the same as the units in the tables.
- Step 2 12 minutes desired time /35 Ft3 = 0.343 minutes per Ft3.
- Step 3 The model SG 1-1/2" unit will evacuate 1 Ft3 in 0.012 minutes, as the performance table for 60 PSIG reads.
- Step 4 0.012 minutes per Ft3 actual/0.343 minutes per Ft3 required = 0.035 Desired Sizing Factor. In this case, choose the SG 1/2" which exceeds the Desired S.F.
- Step 5 The time per Ft3 for the 1-1/2" unit 0.012/0.12 S.F. = 0.100 minutes per Ft3 x 35 Ft3 = 3.5 minutes to evacuate the given volume to 15 In Hg Abs.
- Step 6 The chart says the motive flow (Qm) for an SG 1-1/2" unit is 222 Lb Hr at 60 PSIG motive pressure x 0.12 S.F. = 26.6 Lb/Hr of motive steam required to operate the unit.
Performance for steam-air eductors is determined by tests using gases of a specific molecular weight and temperature. The term Dry Air Equivalent (DAE) is a way of presenting data so that corrections can be made for temperature and molecular weight. The actual gas being pumped will generally be at some temperature and composition other than air at 70°F. As it is not pl1lctical to maintain testing facilities for an infinite number of gases and temperatures of those gases, the method described here has been devised to correct all gases to a standard set of conditions. This allows eductors to be designed under given circumstances, then applied to the actual process conditions they will work under.
This method is described by the Heat Exchange Institute HEI in the book "Standards for Steam Jet Vacuum Systems, Fourth Edition 1988" and is synopsized below. If a more detailed explanation is needed, please refer to the previously mentioned publication.
Calculating average molecular weight of the mixture and finding correction factors
This method deals with the gases in terms of weight of now in a given period of time. The most commonly used is Lb (of gas)/Hr. To proceed, the units of each gas component should be convened to Lb/Hr. (Conversion Factors for several different units are found on the back cover of this brochure.) Water vapor (steam) is handled as a separate component in this calculation because the temperature correction factor is different for condensable gases.
To find the average molecular weight for the gas components, take the Lb/Hr now for each gas component and divide it by the molecular weight of that component. This will result in the number of moles of each component. Then add together the Lb/Hr flows of each component (except the water vapor).
This will result in the total Lb/Hr flow of gas to be pumped. Then add together the total moles of each component. The result will be the total moles of gas to be pumped. Finally, divide the total Lb/Hr flow to be pumped by the total moles to be pumped. The result will be the average molecular weight of the mixture. The average molecular weight of the mixture is then used to obtain a gas weight correction factor from the "Molecular Weight Entrainment Ratio Table" found on page 9 of our Gas Eductors brochure
The correction factor of the water vapor is obtained by reading the correction factor from the "Molecular Weight Entrainment Ratio Table".
For the more common gases the molecular weight of each component may be obtained from this page. If your gas is not here, you can calculate it by adding the weight of the atoms of each element in the gas. (Then if the gas is diatomic, multiply by 2, etc.)
Finding Temperature Correction Factors
Go to the "Temperature Entrainment Ratio Table" on page 9and find the temperature of the suction gas. From the table, obtain the temperature correction factors for gas and steam. These will be put into the correction calculation.
Final Correction Formula
Take the total flow rate (Lb/Hr) of the gases to be pumped minus the flow rate (Lb/Hr) of the water vapor, divide this by the gas flow rate (Lb/Hr) correction factor times the gas temperature correction factor. The result will be the non-condensable gas load for the ejector.
Then take the weight of the water vapor, divide it by the molecular weight correction factor times the temperature correction factor for steam.
Then add the results of these two calculations together. The final result will be the total gas load Lb/Hr required of the eductor in Dry Air Equivalent. This number is used as the desired suction flow (Qs) in the table.
Example:
50 Lb/Hr of a mixed gas and steam at a temperature of 300°F contains 15 Lb/Hr of 02, 10 Lb/Hr of Air, 5 Lb/Hr ofH2, 5 Lb/Hr
Of CO2, and 15 Lb/Hr of steam.
The molecular weights of these gases are as follows:
02 = 32 Lb/Mole
Air = 29 Lb/Mole
H2 = 2 Lb/Mole
CO2 = 44 Lb/Mole
H2o = 18 Lb/Mole
To find the moles of gas per Hr take the actual flow and divide by the Lb/mole
15 Lb/Hr Of O2/32 Lb/Mole = 0.469 Moles/Hr 02
10 Lb/Hr of Air/29 Lb/Mole = 0.345 Moles/Hr Air
5 Lb/Hr of H2/2lb/Mole = 2.500 Moles/Hr H2
5 Lb/Hr of CO2/44 Lb/Mole = 0.114 Moles/Hr CO2
(15 + 10 + 5 +5)/(0.469 + 0.345 + 2.50 +0.114) = 10.21 Average
Mole Weight of mixed gases Gas Molecular Weight Correction Factor for 10.21 = 0.61 (by interpolation)
Steam Molecular Weight Correction Factor for 18 = 0.81 (by interpolation)
Temperature Correction Factor for Gases at 300°F = 0.945
Temperature Correction Factor for Steam at 3OO°F = 0.925
Final Calculation of Example:
(15 + 10 +5 + 5)/(0.61 x 0.945)) + (15/(0.81 x 0.925)) = 80.74 Lb/Hr Dry Air Equivalent.
To choose the correct eductor, pick a unit that will pump 80.74 Lb/Hr of DAE at your desired suction pressure.
Note: Gases with low molecular weight will cause the DAE to be higher than the actual weight being pumped. It is imperative the actual DAE be calculated for gases containing these low molecular weight gases.
